The long-term weight loss effect of Semaglutide
Recently, at the 2024 European Obesity Conference (ECO), Novo Nordisk announced two milestone research results on the weight loss therapy semaglutide (Wegovy). The long-term weight loss effect of semaglutide can be maintained for up to 4 years. No matter of how much weight loss patients lose, their cardiovascular health can be improved. The long-term weight loss efficacy results of semaglutide are simultaneously published in the sub journal Nature Medicine of Nature.
The two studies released this time are based on the analysis results of the SELECT clinical phase 3 trial. The SELECT trial included 17604 overweight or obese adult patients from 41 countries/regions who received weekly subcutaneous injection of 2.4 mg Wegovy or placebo treatment. These patients had previously suffered from heart disease, stroke and/or peripheral artery disease, but did not suffer from type 1 or type 2 diabetes when participating in the study. In 2023, a SELECT trial report showed that after more than 3 years of Wegovy treatment, patients had a 20% reduced risk of death due to heart attack, stroke, or cardiovascular disease, with an average weight loss of 9.4%.
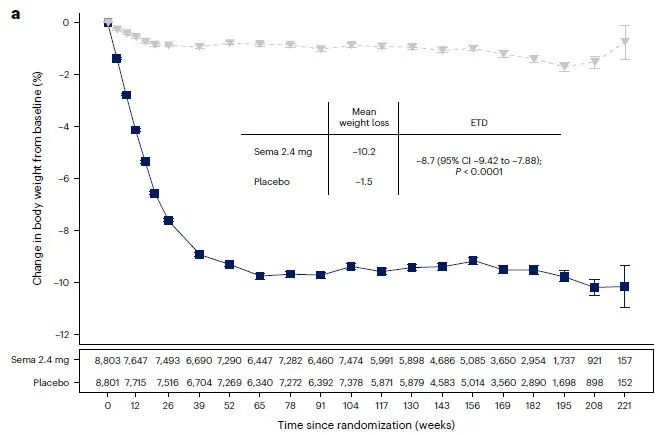
Semaglutide can make overweight or obese adult patients lose weight for more than 65 weeks
The first research released this time shows that Wegovy treatment can make overweight or obese adult patients without diabetes lose weight for more than 65 weeks, and can last for up to 4 years. At week 208, the average weight loss, waist circumference, and waist to height ratio of patients in the Wegovy group decreased by 10.2%, 7.7 cm, and 6.9%, respectively (placebo group patients decreased by 1.5%, 1.3 cm, and 1.0%, respectively; all comparisons P<0.0001). In addition, all patients in the Wegovy group showed clinically significant weight loss, regardless of their gender, race, body size, and place of residence.
It is worth mentioning that after 2 years of treatment, more than half (52%) of patients receiving Wegovy treatment had a decrease in BMI category. For example, obese patients in the Wegovy group (BMI ≥ 30 kg/m) ²). The proportion decreased from 71% to 43%, while the placebo group decreased from 72% to 68%. In addition, 12% of patients in the Wegovy group achieved a healthy weight (BMI ≤ 25 kg/m) ²), The placebo group only had 1.2%.
In terms of security, there is no unexpected security issues. The proportion of serious adverse events (SAEs) in the Wegovy group was lower than that in the placebo group (33% vs 36%). More patients receiving Wegovy treatment withdrew from the trial due to gastrointestinal symptoms (including nausea and diarrhea), mainly during the 20 week dose escalation phase. In addition, in various BMI categories (<30, 30 to<35, 35 to<40, and ≥ 40 kg/m) ²) Compared with placebo, the incidence of serious adverse events was lower with Wegovy (43.23, 43.54, 51.07, and 47.06 for Wegovy, 50.48, 49.66, 52.73, and 60.85 for placebo). Wegovy did not increase the incidence rate of pancreatitis, but the incidence rate of gallstones was higher in Wegovy group.
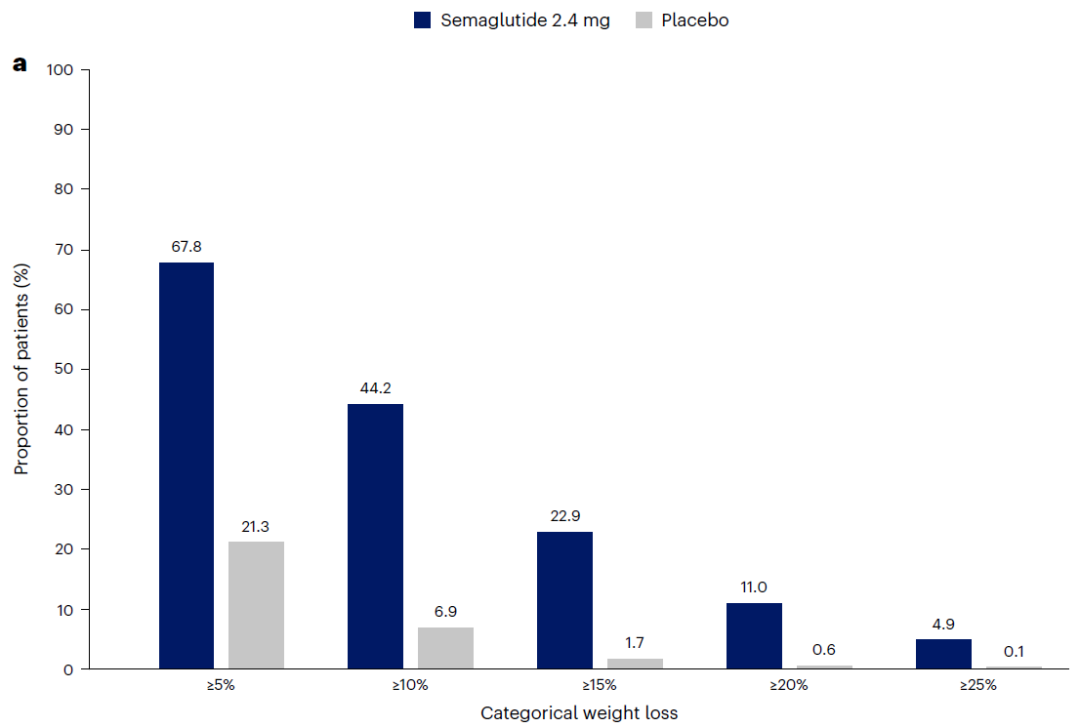
The long-term weight loss effect of semaglutide
Semaglutide may impact the public health burden of various obesity related diseases
“The adult overweight and obese people without diabetes, clinically relevant weight loss can be maintained for up to 4 years. Such a large and diverse population has this degree of weight loss. Semaglutide may have the potential to impact the public health burden of various obesity related diseases. Although our experiment focuses on cardiovascular events, many other chronic diseases, including several types of cancer, osteoarthritis, anxiety and depression, may also benefit from effective weight management Professor Donna Ryan from the Pennington Biomedical Research Centre in New Orleans, USA, led the study on the long-term weight loss effects of Wegovy.
The second study released this time examined the relationship between patient weight indicators at baseline, weight changes during the study period, and cardiovascular outcomes, including the time of first major adverse cardiovascular event (MACE) and heart failure. MACE includes cardiovascular death, non fatal myocardial infarction, or non fatal stroke.
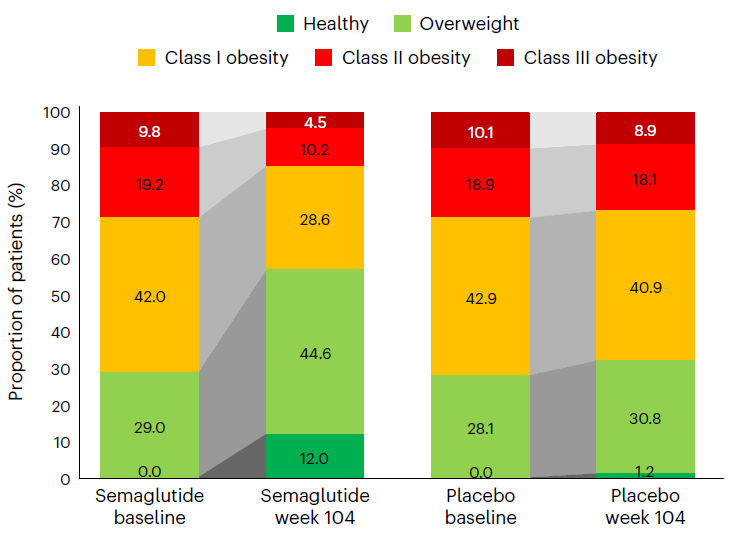
Compared to baseline, after receiving semaglutide treatment for 104 weeks, the patient’s BMI category decreased
The second study released this time examined the relationship between patient weight indicators at baseline. Weight changes during the study period, and cardiovascular outcomes, including the time of first major adverse cardiovascular event (MACE) and heart failure. MACE includes cardiovascular death, non fatal myocardial infarction, or non fatal stroke.
The research results show that regardless of the patient’s weight and weight loss at baseline, Wegovy treatment brings cardiovascular benefits. That is to say, even patients with mild obesity or those with minimal weight loss can improve their cardiovascular disease prognosis.
Semaglutide also have other cardiovascular risk reducing effects besides reducing unhealthy body fat
“These findings have significant clinical implications. I have observed in clinical practice that approximately half of cardiovascular disease patients’ weight is equivalent to the level of patients in the SELECT trial. They are likely to benefit from Wegovy treatment.” Professor John Deanfield from University College London said. “Our findings suggest that the magnitude of this treatment effect is not related to the amount of weight loss, indicating that the drug has other cardiovascular risk reducing effects besides reducing unhealthy body fat. These alternative mechanisms may include positive effects on blood sugar, blood pressure, or inflammation, as well as direct effects on myocardium and blood vessels, or a combination of these factors.” Professor Deanfield led this study.
The positive results announced this time provide strong evidence for Wegovy’s long-term weight loss effect. Its benefits in preventing cardiovascular disease. However, researchers also remind that, SELECT is not a primary prevention trial in experimental design. Furthermore, despite the large and diverse sample size of participants in the trial, it did not include enough individuals from different ethnic groups, making it difficult to infer the effectiveness of the drug in patients of different ethnicities.